Clinacox®
If coccidiosis is a major problem with your poultry, knock it down with Clinacox®, the one-cycle solution for getting Intestinal Integrity back on track. Available from Elanco, proven and powerful Clinacox gives a fresh start to your cocci control program if you’re experiencing waning effectiveness.
Key features
- Strong, Effective Treatment — Clinacox cleans up stubborn coccidiosis and helps improve your poultry operation’s Intestinal Integrity (I2) score.
- Use in Sequence with Other Ionophores or Chemicals — Use Clinacox for one cycle to reset coccidiosis management and follow it with other ionophores or chemicals to maintain long-term coccidiosis control.
- Use Clinacox Judiciously — Due to Clinacox’s mode of action against cocci parasites, partner with your Elanco technical consultant to manage your program appropriately.
Clinacox Directions for use:
Broiler chickens: For the prevention of coccidiosis caused by Eimeria tenella, E. necatrix, E. acervulina,
E. brunetti, E. mitis (mivati) and E. maxima.
Because diclazuril is effective against E. maxima later in its life cycle, subclinical intestinal lesions may be present for a short time after infection. Diclazuril was shown in studies to reduce lesion scores and improve performance and health of birds challenged with E. maxima.
Growing Turkeys: For the prevention of coccidiosis caused by Eimeria adenoeides, E. gallopavonis and E. meleagrimitis.
To manufacture a Type C medicated feed directly from the Type A medicated article: Thoroughly mix one pound (1 lb) of CLINACOX (0.2% diclazuril) into each ton of complete feed to provide 0.91 g/ton (1 ppm) of diclazuril (use level). It is recommended that an intermediate mix containing one part Clinacox and not less than nine parts appropriate feed ingredient be thoroughly mixed before incorporation into the final feed.
To manufacture a Type B medicated feed and then a Type C medicated feed: Thoroughly mix 200 lbs of Clinacox (0.2% diclazuril) with 1800 lbs unmedicated feed to produce 2000 lbs of Type B medicated feed containing 182 g/ton (200 ppm) diclazuril. Thoroughly mix 10 lbs of the resulting Type B medicated feed with 1990 lbs of unmedicated feed to produce 2000 lbs of Type C medicated feed containing 0.91 g/ton (1 ppm) diclazuril.
The resulting Type C medicated feed should be fed continuously as the sole ration.
CAUTION:
Do not feed to breeding turkeys.
No withdrawal period is required when used according to labeling. Do not feed to birds producing eggs for human consumption.
Coban directions for use:
As an aid in the prevention of coccidiosis caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima in broiler chickens:
- Feed Coban at 90-110 g/ton
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label)
For the prevention of coccidiosis in turkeys caused by Eimeria adenoeides, E. meleagrimitis and E. gallopavonis:
- Feed Coban at 54-90 g/ton to turkeys
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label)
Important Safety Information
WARNING: Do not feed to laying chickens. Do not feed to chickens over 16 weeks of age.
CAUTION: Do not allow horses, other equines, mature turkeys, or guinea fowl access to feed containing monensin. Ingestion of monensin by horses and guinea fowl has been fatal. Some strains of turkey coccidia may be monensin tolerant or resistant. Monensin may interfere with development of immunity to turkey coccidiosis. In the absence of coccidiosis in broiler chickens the use of monensin with no withdrawal period may limit feed intake resulting in reduced weight gain. Not for replacement chickens intended to become broiler breeding chickens.
Inteprity directions for use:
For the prevention of mortality caused by necrotic enteritis associated with Clostridium perfringens in broiler chickens:
- Avilamycin is to be fed at 13.6 to 40.9 grams per ton of Type C medicated feed (15 to 45 ppm) as the sole ration for 21 consecutive days
- Feed to chickens that are at risk of developing, but not yet showing clinical signs of, necrotic enteritis associated with Clostridium perfringens
Inteprity Important Safety Information
CAUTION: Federal law restricts medicated feed containing this Veterinary Feed Directive (VFD) drug to use by or on the order of a licensed veterinarian. To assure responsible antimicrobial drug use in broiler chickens, treatment administration must begin on or before 18 days of age. When using in combination with Maxiban, Monteban, Coban and BioCox, treatment and administration must begin on or before 18 days of age.
The safety of avilamycin has not been established in chickens intended for breeding purposes.
Avilamycin has not been demonstrated to be effective in broiler chickens showing clinical signs of necrotic enteritis prior to the start of medication.
The Veterinary Feed Directive (VFD) expiration date must not exceed 90 days from the date of issuance. VFDs for avilamycin shall not be refilled.
Maxiban directions for use:
For the prevention of coccidiosis in broiler chickens caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima:
- Feed Maxiban at 54-90 g/ton
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label), some combination use requires 5-day withdrawal
CAUTION: Nicarbazin medicated broilers may show reduced heat tolerance if exposed to high temperature and high humidity. Provide adequate drinking water and ventilation. Do not allow adult turkeys, horses or other equines access to formulations containing narasin. Ingestion of narasin by these species has been fatal. Do not feed to laying hens.
Monteban directions for use:
For the prevention of coccidiosis caused by Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati and E. maxima in broiler chickens:
- Feed Monteban at: 54–90 g/ton
- Feed continuously as the sole ration
- Requires a zero-day withdrawal (when fed according to the label)
CAUTION: Do not allow adult turkeys, horses or other equines access to narasin formulations. Ingestion of narasin by these species has been fatal.
ZoaShield Directions for Use:
Indications
For use in preparation of feeds for chickens and turkeys only. Livestock remedy.
- Broiler Chickens: For prevention and control of coccidiosis.
- Replacement Chickens: For development of active immunity to coccidiosis.
- Growing Turkeys: For prevention and control of coccidiosis.
Directions
It is suggested that a mixture of ZoaShield and some feed ingredient be prepared prior to mixing in with the finished ration. This will ensure thorough and even distribution in the feed.
For chickens grown for meat purposes:
Use 1 lb. (454 g) of ZoaShield per 2,000 lb. (909 kg) of finished product to produce a feed containing 0.0125% zoalene. Feed containing zoalene should be fed continuously as the only ration from the time chicks are placed in floor pens until they are slaughtered for meat.
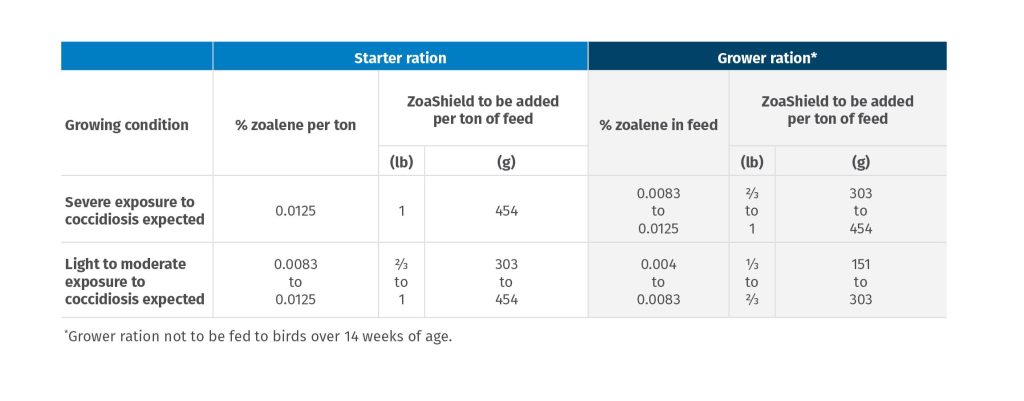
For replacement chickens:
When used under conditions of exposure to coccidiosis, zoalene will allow immunity to develop adequately to protect against losses due to the disease when the birds are placed on non-medicated feed for egg laying purposes. The following chart outlines the type of feeding program to follow where completely formulated feed is the sole ration:
The State of Salmonella in the Poultry Industry
There are several factors that can make controlling Salmonella in poultry a difficult task. Chief among them is the long-standing perception held by some operations that processing plant interventions can eliminate most of the risk associated with any potential threat.
Processing has traditionally been viewed as the last line of defense for safeguarding consumers from food-borne disease. But for integrated companies, it’s important that the entire organization understands the role it plays in helping the plant deliver the safest product possible.
What can be unclear is who in the poultry production chain is responsible for controlling Salmonella and how all roles in the poultry industry must work together to further reduce its presence in poultry products. To help the poultry industry better understand industry perceptions of Salmonella and its control, Elanco Animal Health fielded a blind industry survey to identify steps the poultry industry is taking to control Salmonella and who the industry believes is ultimately responsible for the control of Salmonella. The survey was distributed to live-side production, food safety and quality assurance professionals in the poultry industry.
Who is Responsible for Salmonella Control?1
All respondents agree that Salmonella reduction is a shared responsibility across every production stage. However, there is a gap in belief about who is ultimately responsible for Salmonella control.
Respondents with QA/food safety roles completely agree that live-side production is the first step in Salmonella control and more emphasis needs to be placed on the pre-harvest prevention of Salmonella. However, those in live-side production roles agree to a far lesser extent. While live-side production believes Salmonella control is a shared responsibility, their opinions differ from QA on the importance of pre-harvest interventions.
For turkeys grown for meat purposes only:
When turkey poults are reared in confinement and severe exposure to coccidiosis is usually a problem, use 1½ lb. (681 g) of ZoaShield per ton (2,000 lb.) of feed to produce a finished feed containing 0.0187% zoalene. Under the usual conditions of rearing turkey poults, or when turkey poults are on range, use 1 lb. (454 g) of ZoaShield per 2,000 lb. (909 kg) of feed to produce a finished feed containing 0.0125% zoalene. The feed containing zoalene should be fed continuously until the birds are 14 to 16 weeks of age.
Cautions
Not to be fed to laying birds.
Consult a veterinarian or poultry pathologist if losses exceed 0.5% in a two-day period.



